INDICATION AND IMPORTANT SAFETY INFORMATION
WARNING: HEPATIC DECOMPENSATION AND FAILURE IN PRIMARY BILIARY CHOLANGITIS PATIENTS WITH CIRRHOSIS
- Hepatic decompensation and failure, sometimes fatal or resulting in liver transplant, have been reported with OCALIVA treatment in primary biliary cholangitis (PBC) patients with either compensated or decompensated cirrhosis.
- OCALIVA is contraindicated in PBC patients with decompensated cirrhosis, a prior decompensation event, or with compensated cirrhosis who have evidence of portal hypertension.
- Permanently discontinue OCALIVA in patients who develop laboratory or clinical evidence of hepatic decompensation; have compensated cirrhosis and develop evidence of portal hypertension; or experience clinically significant hepatic adverse reactions while on treatment.
INDICATION
OCALIVA, a farnesoid X receptor (FXR) agonist, is indicated for the treatment of adult patients with primary biliary cholangitis (PBC)
- without cirrhosis or
- with compensated cirrhosis who do not have evidence of portal hypertension,
either in combination with ursodeoxycholic acid (UDCA) with an inadequate response to UDCA or as monotherapy in patients unable to tolerate UDCA.
This indication is approved under accelerated approval based on a reduction in alkaline phosphatase (ALP). An improvement in survival or disease-related symptoms has not been established. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
Contraindications
OCALIVA is contraindicated in patients with:
- decompensated cirrhosis (e.g., Child-Pugh Class B or C) or a prior decompensation event.
- compensated cirrhosis who have evidence of portal hypertension (e.g., ascites, gastroesophageal varices, persistent thrombocytopenia).
- complete biliary obstruction.
Warnings and Precautions
Hepatic Decompensation and Failure in PBC Patients with Cirrhosis
Hepatic decompensation and failure, sometimes fatal or resulting in liver transplant, have been reported with OCALIVA treatment in PBC patients with cirrhosis, either compensated or decompensated. Among postmarketing cases reporting it, median time to hepatic decompensation (e.g., new onset ascites) was 4 months for patients with compensated cirrhosis; median time to a new decompensation event (e.g., hepatic encephalopathy) was 2.5 months for patients with decompensated cirrhosis. Some of these cases occurred in patients with decompensated cirrhosis when they were treated with higher than the recommended dosage for that patient population; however, cases of hepatic decompensation and failure have continued to be reported in patients with decompensated cirrhosis even when they received the recommended dosage.
Hepatotoxicity was observed in the OCALIVA clinical trials. A dose-response relationship was observed for the occurrence of hepatic adverse reactions including jaundice, worsening ascites, and primary biliary cholangitis flare with dosages of OCALIVA of 10 mg once daily to 50 mg once daily (up to 5-times the highest recommended dosage), as early as one month after starting treatment with OCALIVA in two 3-month, placebo-controlled clinical trials in patients with primarily early stage PBC.
Routinely monitor patients for progression of PBC, including hepatic adverse reactions, with laboratory and clinical assessments to determine whether drug discontinuation is needed. Closely monitor patients with compensated cirrhosis, concomitant hepatic disease (e.g., autoimmune hepatitis, alcoholic liver disease), and/or with severe intercurrent illness for new evidence of portal hypertension (e.g., ascites, gastroesophageal varices, persistent thrombocytopenia) or increases above the upper limit of normal in total bilirubin, direct bilirubin, or prothrombin time to determine whether drug discontinuation is needed. Permanently discontinue OCALIVA in patients who develop laboratory or clinical evidence of hepatic decompensation (e.g., ascites, jaundice, variceal bleeding, hepatic encephalopathy), have compensated cirrhosis and develop evidence of portal hypertension (e.g., ascites, gastroesophageal varices, persistent thrombocytopenia), experience clinically significant hepatic adverse reactions, or develop complete biliary obstruction. If severe intercurrent illness occurs, interrupt treatment with OCALIVA and monitor the patient’s liver function. After resolution of the intercurrent illness, consider the potential risks and benefits of restarting OCALIVA treatment.
Severe Pruritus
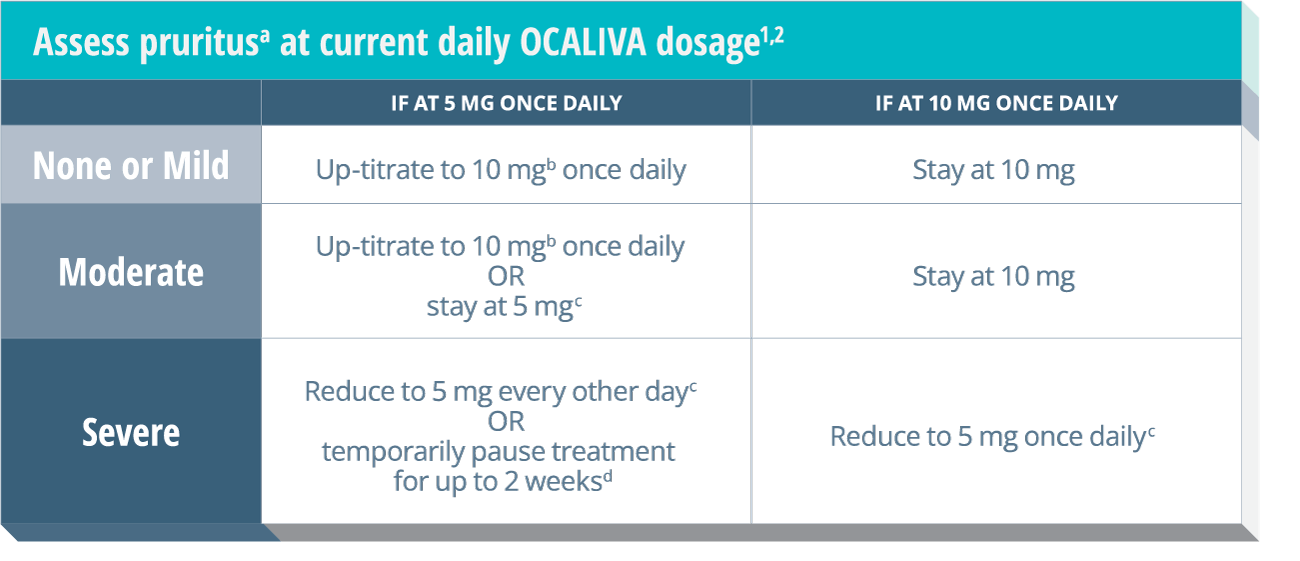
Severe pruritus was reported in 23% of patients in the OCALIVA 10 mg arm, 19% of patients in the OCALIVA titration arm, and 7% of patients in the placebo arm in a 12-month double-blind randomized controlled clinical trial of 216 patients. Severe pruritus was defined as intense or widespread itching, interfering with activities of daily living, or causing severe sleep disturbance, or intolerable discomfort, and typically requiring medical interventions. Consider clinical evaluation of patients with new onset or worsening severe pruritus. Management strategies include the addition of bile acid binding resins or antihistamines, OCALIVA dosage reduction, and/or temporary interruption of OCALIVA dosing.
Reduction in HDL-C
Patients with PBC generally exhibit hyperlipidemia characterized by a significant elevation in total cholesterol primarily due to increased levels of high-density lipoprotein-cholesterol (HDL-C). Dose-dependent reductions from baseline in mean HDL-C levels were observed at 2 weeks in OCALIVA-treated patients, 20% and 9% in the 10 mg and titration arms, respectively, compared to 2% in the placebo arm. Monitor patients for changes in serum lipid levels during treatment. For patients who do not respond to OCALIVA after 1 year at the highest recommended dosage that can be tolerated (maximum of 10 mg once daily), and who experience a reduction in HDL-C, weigh the potential risks against the benefits of continuing treatment.
Adverse Reactions
The most common adverse reactions (≥5%) are: pruritus, fatigue, abdominal pain and discomfort, rash, oropharyngeal pain, dizziness, constipation, arthralgia, thyroid function abnormality, and eczema.
Drug Interactions
Bile Acid Binding ResinsBile acid binding resins such as cholestyramine, colestipol, or colesevelam adsorb and reduce bile acid absorption and may reduce the absorption, systemic exposure, and efficacy of OCALIVA. If taking a bile acid binding resin, take OCALIVA at least 4 hours before or 4 hours after taking the bile acid binding resin, or at as great an interval as possible.
WarfarinThe International Normalized Ratio (INR) decreased following coadministration of warfarin and OCALIVA. Monitor INR and adjust the dose of warfarin, as needed, to maintain the target INR range when co-administering OCALIVA and warfarin.
CYP1A2 Substrates with Narrow Therapeutic IndexObeticholic acid may increase the exposure to concomitant drugs that are CYP1A2 substrates. Therapeutic monitoring of CYP1A2 substrates with a narrow therapeutic index (e.g., theophylline and tizanidine) is recommended when co-administered with OCALIVA.
Inhibitors of Bile Salt Efflux PumpAvoid concomitant use of inhibitors of the bile salt efflux pump (BSEP) such as cyclosporine. Concomitant medications that inhibit canalicular membrane bile acid transporters such as the BSEP may exacerbate accumulation of conjugated bile salts including taurine conjugate of obeticholic acid in the liver and result in clinical symptoms. If concomitant use is deemed necessary, monitor serum transaminases and bilirubin.
Please click here for Full Prescribing Information, including Boxed WARNING.
To report SUSPECTED ADVERSE REACTIONS, contact Intercept Pharmaceuticals, Inc. at 1-844-782-ICPT or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
If you have questions or would like more information about OCALIVA, contact Intercept Medical Information.